Secondary organic mass formation from night-day processing of cresol oxidation products
Anke Mutzel, Olaf Böge and Hartmut Herrmann
The chemistry that takes place during the daytime influences the subsequent nighttime chemistry and vice versa. Compounds that are emitted during the daytime are continuously oxidized by OH radicals or ozone to form semi-volatile organic compounds (SVOCs). In the evening when the OH radical production drops down, VOCs and their oxidation products that remain in the atmosphere are subjected to the nighttime chemistry in which the NO3 radicals and ozone are most important. Equally, the daytime chemistry processes VOCs and SVOCs that are emitted or formed during the nighttime. Therefore, daytime and nighttime chemistry cannot be considered separately but strongly influence each other. So far, laboratory SOA formation studies have focused on either daytime chemistry or nighttime chemistry, and the knowledge about their interaction and their influence on the fate of VOCs, SVOCs and SOA formation are generally missing.
In the present study, experiments were conducted to investigate the interaction of day- and nighttime chemistry. The oxidation of m-cresol was selected as precursor compound. Two sets of experiments were conducted. Experiment type A starts with daytime chemistry (OH radical) followed by nighttime chemistry (NO3 radical) and the experiments type B start with nighttime chemistry (NO3 radical) with a subsequent daytime chemistry (OH radical). The relative humidity was varied in three steps (0, 50, 75%). The experiments were conducted in the presence of slightly acidic seed particles (pH=4).
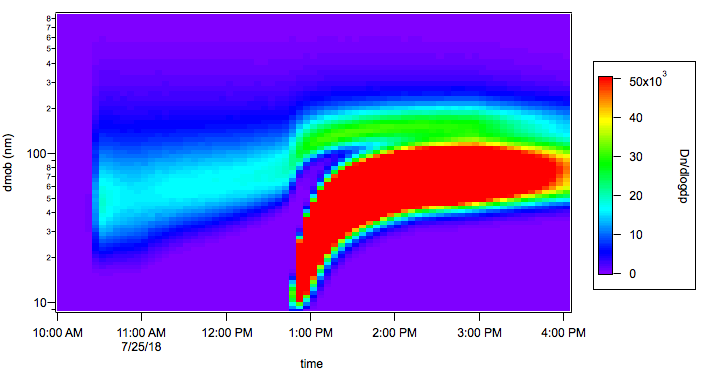
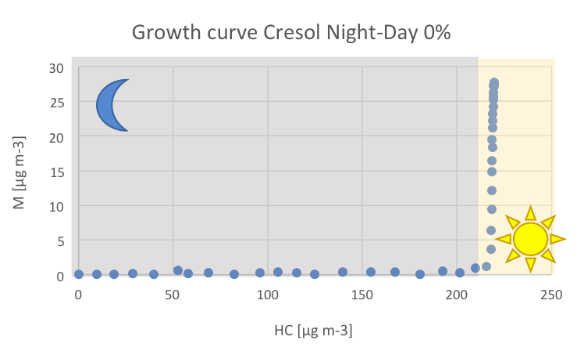
Figure 1. Nucleation event occurring during night-day processing of m-cresol. Nucleation starts after changing from night- to daytime chemistry (A). The strong organic mass formation can also be seen from the growth curve (B ).
The experiments clearly demonstrated that the night-day processing of m-cresol leads to a massive formation of secondary mass while changing from night- to day-chemistry. During nighttime, no SOA formation can be observed. When changing from night- to daytime chemistry and after all m-cresol is consumed, the organic mass formation starts. This leads
to an increase of organic mass of 28 µg m-3 solely formed by secondary processes of nocturnal oxidation products.
PTR-MS and C-TOF revealed the occurrence of two groups of compounds that are related to methyl benzoquinone structure and to nitro-cresol structure, respectively. The secondary formation is most likely attributed to the partitioning/further reaction of the latter class of compounds because very strong correlation to the increase of organic mass (> 0.9) was found. As this was not observed from day-night experiments, the present results highlight the importance of nighttime oxidation of anthropogenic VOCs emitted in the evening or remaining from previous day processing. The further oxidation of such compounds during night leads to gas-phase oxidation products that act as reservoir compounds which then very efficiently trigger SOA formation during the early morning hours of the next day.
